On July 20, 2022, Health Canada published amendments to the Food and Drug Regulations ("FDR") creating a requirement for prepackaged foods whose contents meet or exceed certain thresholds of saturated fat, sodium and/or sugars to include a symbol on the front label of their packages.
The amended regulations come into force immediately and follow the announcement from the Minister of Health on June 30. Manufacturers will have until December 31, 2025 to change labels on prepackaged foods to comply with the new requirements.
Health Canada published these regulations under its mandate to promote the health and safety of Canadians. The introduction of thresholds that trigger the requirement for a FOP nutrition symbol indicating a "high" quantity of saturated fat, sodium and/or sugar is meant to help Canadian consumers make informed choices about their prepackaged food purchases.
FOP nutrition symbol
Prepackaged products that meet or exceed prescribed thresholds for saturated fat, sodium and/or sugar are now required to carry a FOP nutrition symbol on the label's principal display panel to indicate that the food is high in one or more of those nutrients. The FOP nutrition symbol is meant to complement the Nutrition Facts table, which is found on the back of food packages.
The FOP nutrition symbol consists of a black and white magnifying glass, which is meant to draw consumers' attention to the notice that the product is "High in" saturated fat, sodium and/or sugar. The "Directory of Nutrition Symbol Specifications," incorporated by reference into the FDR, will be published online and will set out specifications for use. The FOP nutrition symbol must be bilingual (either one symbol in English and one symbol in French, or one symbol in both languages) and must include the words: "Health Canada" at the bottom. For consistency, the symbol must appear in the upper half of the label, depending on its shape and size.
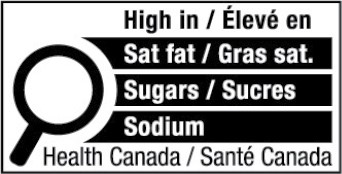
Applicable DV Thresholds
Generally, prepackaged foods that contain saturated fat, sodium and/or sugar at levels that meet or exceed 15% of the Daily Value ("DV") will be required to display the FOP nutrition symbol. Prepackaged foods with a smaller reference amount, where the concentration of saturated fat, sodium and/or sugar is higher per serving, will be subject to a lower threshold (10% DV). Prepackaged foods that are main dishes with a greater reference amount, and which make up more of a consumer's daily intake of nutrients, will be subject to a higher threshold (30% DV).
New claims and limitations on nutrient content claims
The amended regulations also incorporate by reference an updated "Table of Permitted Nutrient Content Statements and Claims," which outlines the conditions of use for various nutrient content claims. This updated table includes some new and revised claims (e.g. lower in sugar claim, changes to criteria for sugar-free claim).
The use of a nutrient content claim related to saturated fat, sodium or sugar on the principal display panel of a label is prohibited when a food displays a nutrition symbol that identifies the food as being "high in" that particular nutrient. Similarly, the use of the "unsweetened" claim is prohibited on the principal display panel of foods displaying a "high in sugars" nutrition symbol.
Exemptions and prohibitions
Health Canada has carved out some exemptions from the new regulatory requirements. Some foods—such as raw whole ground meats and alcoholic beverages—are conditionally exempt as long as they continue to meet the conditions that enable their exemption status. Other foods—such as fresh vegetables, whole eggs and milk from any animal—are exempt for having recognized health benefits. Other exemptions include, for example, products whose labels have a very small surface area, foods where a nutrition symbol would be redundant, like honey or table salt, and prepackaged products that are served in commercial enterprises, such as restaurants. Finally, cheese and yogurt are conditionally exempt on the basis that they are high in calcium. This exemption will be reassessed after ten years.
In addition to the exemptions, certain foods are prohibited from carrying the FOP nutrition symbol. Categories of food for special dietary use—such as meal replacements, nutritional supplements and human milk substitutes—are prohibited from carrying the symbol. This prohibition does not include gluten-free foods. Additionally, foods targeted to infants between the ages of six months and one year of age are also prohibited from carrying the symbol.
Other amendments
In addition to the FOP nutrition labelling requirement, Health Canada has made other amendments to the FDR, repealing certain labelling requirements for foods containing high-intensity sweeteners while clarifying that foods sweetened with aspartame are still required to display a statement to the effect that aspartame contains phenylalanine. Additionally, to further Health Canada's ban on the use of partially hydrogenated oils ("PHOs"), references to PHOs have been removed from the FDR and the definitions of "hydrogenated" and "partially hydrogenated oils" have been revised. Finally, in recognition that Canadians' vitamin D levels typically range from insufficient to deficient, the amended regulations increase the amounts of vitamin D that are required to be added to cow's milk and margarine, and permitted to be added to goat's milk.
***
When combined, these regulatory changes can pose unique challenges to food brands and advertisers who may not otherwise appreciate the impact of these changes. Gowling WLG's Food and Beverage Group, includes professionals with expertise in regulatory, advertising and trademarks who can help make sense of this evolving landscape, and how these changes impact your business.
The authors would like to thank Victoria Asikis, Associate, and Priya Szymanski, Summer Law Student, for their assistance in publishing this article.